The openness of SIP has allowed it to attract resources from around the globe, which has laid a good foundation for the development of the medical device industry.

Double wheels to drive the industry
In SIP, opening-up and innovation are well integrated. International cooperation has allowed multinationals to accelerate localization in SIP, and local enterprises to gain advanced concepts, technologies and experience.
The cooperation between Johnson & Johnson and Chinese Academy of Sciences’ Suzhou Institute of Nano-tech and Nano-bionics in medical coating technology has entered the third phase, and both Eli Lilly and Company and Danaher have made investments in biomedicine enterprises in SIP.

Efforts to build an assisted reproductive technology industry chain

Basecare, founded in SIP in 2010, has brought into market its new testing kit for preimplantation genetic screening (PGS) aimed at detecting chromosome abnormalities of IVF (in-vitro fertilization) embryos. The company is striving to build an assisted reproductive technology industry chain.
Last June, Basecare acquired Genea Biomedx, world-famous fertility group Genea’s high-end medical devices arm in Singapore. And one month later, it signed an agreement with Genea to launch strategic cooperation in global marketing and introduction of advanced products to China.
According to CFO Yin Lejun, in addition to accelerating R&D and manufacturing of its own products, the company would enhance efforts to integrate upstream and downstream resources through SIP’s platforms to propel stronger development of the assisted reproductive technology industry.
Accelerating clinical application of new products
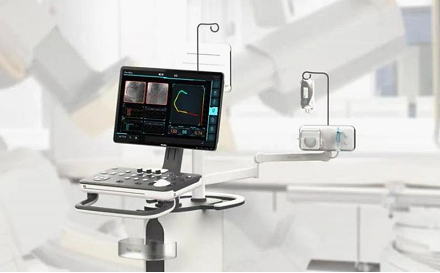
In this aspect, Suzhou Rainmed Medical Technology Co Ltd, which was founded in 2014, set a good example. Based on its advanced medical imaging algorithms, fluid dynamics analysis, mechanical design and R&D of interventional consumables, it has developed a pipeline of industry-leading products.
Suzhou Rainmed Medical Technology has made success in commercializing its FlashAngio caFFR System, a coronary angiography-derived fractional flow reserve measurement system, and FlashAngio caIMR System, a device for measurement of coronary angiography-derived index of microvascular resistance (caIMR).

The company has established partnerships with many hospitals to cooperate in commercializing its products. So far, its products have been used by over 1,300 hospitals in 30 provincial-level regions in China and 15 other countries and regions.







